木质素臭氧氧化降解动力学和机理探讨
来源:wenku163.com 资料编号:WK1634669 资料等级:★★★★★ %E8%B5%84%E6%96%99%E7%BC%96%E5%8F%B7%EF%BC%9AWK1634669
资料介绍
木质素臭氧氧化降解动力学和机理探讨(7600字)
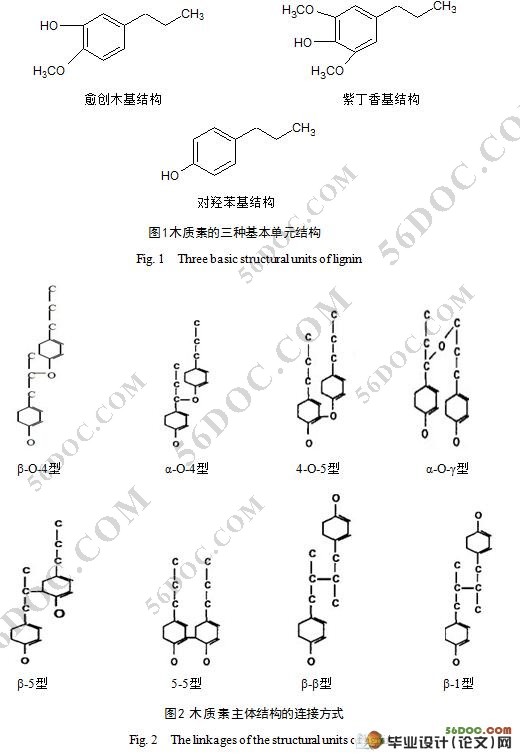
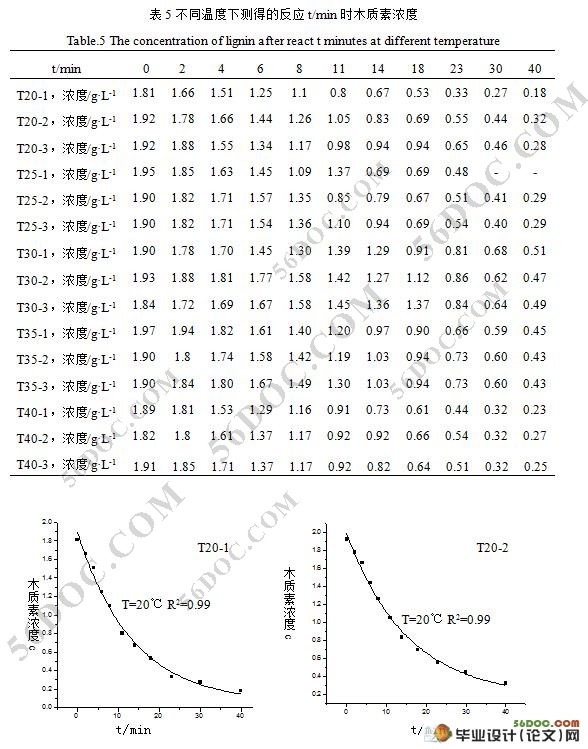
摘 要:本实验将纯木质素溶于氢氧化钠溶液中,采用臭氧分别在不同温度下(20℃,25℃,30℃,35℃ ,40℃)氧化降解木质素,整个实验过程保持臭氧浓度恒定且相对过饱和,研究了该反应在不同温度下的反应速率常数和反应级数。利用紫外分光光度计测定氧化前后木质素溶液的吸收光谱,初步探讨了反应机理。结果表明,木质素臭氧氧化动力方程可表示为:dc/dt=-kcn,其反应级数(n)为一级。经臭氧氧化后木质素的苯环结构遭到破坏,据文献其可能氧化分解为有机羧酸和醛同等小分子化合物。
关键词:木质纤维素;臭氧;动力学;反应机理,预处理
Kinetic and mechanism study of the degradation of lignin by ozone
Abstract: Lignocellulose has attract lots of researchers due to its widely growth in our world.The kinetic and mechanism of the degradation of lignin by ozone was investigated in this aticle.The lignin wh- ich was dissolved in sodium hydroxide (NaOH) solution was oxide by ozone at 20℃,25℃,30℃,35℃,
40℃,respetively. The constant of reaction rate and reaction order has been confirmed.The result showed that the degradation kinetic model of lignin with following form:dc/dt=-kcn,the reaction is a first reaction. This article uses ultraviolet to study the mechinsm of the degradation of lignin by ozone,the spectrum sho- ws that the lignin structure of benzene rings was oxidation into low organic compound such as carboxylic acid ,aldehyde and ketone.
Key word:Lignocellulose; ozone; kinetic; mechanism; pretreatment
|





